Cobalt is an extremely important strategic metal, mainly used in cemented carbide, ceramic color glaze, battery material, superalloy and magnetic material and other fields. In recent years, as the country vigorously develops the new energy industry, the cobalt smelting industry has developed vigorously, and the market demand for cobalt raw materials has become increasingly strong.
Africa's Congo (Kinshasa) is rich in copper and cobalt resources, and its cobalt reserves account for nearly 50% of the world's total reserves. In recent years, more and more cobalt smelting and production enterprises in my country have built local factories in the Democratic Republic of the Congo to process and produce crude cobalt salts in order to reduce production costs and raw material transportation costs. It has gradually become an industry trend to replace the import of cobalt concentrate with crude cobalt salt.
The currently commonly used crude cobalt salt production process has a series of shortcomings such as unsatisfactory impurity removal effect, high consumption of auxiliary materials, and low cobalt grade of the product. It is urgent to develop a new process or optimize the existing crude cobalt salt production process to reduce production. cost, improve product quality and enhance product competitiveness.
In this paper, a cobalt-containing and low-copper raffinate in Congo (Kinshasa) was used as raw material, and high-grade crude cobalt hydroxide was prepared through the process of impurity removal-first-stage cobalt precipitation-second-stage cobalt precipitation-filtration washing-flash drying. Different from the usual process of returning the second-stage cobalt underflow to the impurity removal or leaching process to ensure product quality, this paper returns all the second-stage cobalt underflow to the first-stage cobalt as reaction seeds, thereby greatly improving the recovery rate of cobalt and increasing the crude oil. The output and comprehensive recovery rate of cobalt hydroxide products, and its cobalt content is over 39%.
1 Raw material equipment and test method
1.1 Raw materials for production
The cobalt-containing raffinate used in the production is a low-copper cobalt sulfate solution obtained from a copper-cobalt ore in the Democratic Republic of the Congo after grinding, leaching, and extraction. The main chemical components are shown in Table 1.
Table 1 Main chemical components of cobalt-containing and low-copper raffinate/(g L-1)
1.2 Main production accessories
Quicklime: Industrial grade, -74μm particle size accounted for not less than 80%, active calcium oxide main content not less than 75%.
Sodium metabisulfite: industrial grade, -74μm particle size accounts for not less than 70%, and the main content is not less than 93%.
Magnesium Oxide: Industrial grade, -74μm particle size accounts for not less than 90%, the main content is not less than 95%, and the activity is not less than 85%.
Compressed air: industrial grade, the air pressure is not lower than 0.4MPa.
1.3 Sample analysis instruments and equipment
X-ray fluorescence spectrometer, flame atomic absorption spectrophotometer, inductively coupled plasma spectrometer, potentiometric titrator, electronic balance, digital display pH meter and acid-base titration platform, etc.
1.4 Test principle
The treatment process mainly consists of processes such as impurity removal and cobalt precipitation. Impurity removal is by adding a certain proportion of sodium pyrosulfite slurry and compressed air to the low-copper and cobalt raffinate to adjust the reaction redox environment, and oxidize the Fe2+ that is difficult to hydrolyze and precipitate into Fe3+ that is easier to hydrolyze and precipitate, and at the same time pass through the quicklime slurry The pH value of the solution is adjusted by the material to achieve the purpose of removing most of the iron ions. In addition, impurity ions such as Cu and Mn can also be partially removed during the impurity removal process. Precipitation of cobalt is mainly done by adding active magnesium oxide slurry, controlling the pH value of the reaction, so that the Co ions in the solution are precipitated as Co(OH)2 precipitates, and then through the process of filter press slurry washing, flash drying and other processes to obtain a water content of about 8 % cobalt hydroxide dry product.
1.4.1 Removal of impurities
The relevant chemical reactions involved in the impurity removal reaction are as follows:
1.4.2 Cobalt precipitation reaction
The relevant chemical reactions involved in cobalt deposition are as follows:
2 Test results and discussion
2.1 Impurity removal process conditions
2.1.1 Effect of limestone slurry concentration on impurity removal effect
Set the impurity removal time to 5h, pH = 4.0, potential 420mV, and the influence of quicklime preparation concentration on the impurity removal effect and cobalt ion loss rate are shown in Figures 1 and 2 respectively. It can be seen from Figure 1 that the preparation concentration of quicklime has little effect on the removal effect of impurity ions. As the concentration increases, the removal rate of iron ions is stable above 99%, and the removal rate of manganese ions basically fluctuates between 35% and 42%. The copper ion removal rate fluctuates between 50% and 60%.
It can be seen from Figure 2 that as the concentration of quicklime increases, the loss rate of cobalt for removal of impurities also gradually increases. When the concentration reaches 30%, the loss rate of cobalt reaches 12.6%. The more quicklime auxiliary materials added to the space, the more easily the impurity removal reaction will be partially over-alkaline, and a large amount of cobalt ions will precipitate out instantly, and the cobalt hydroxide precipitate will be wrapped by calcium iron slag and cannot be returned to dissolve and remove impurities solution, resulting in the loss of cobalt ions during the impurity removal process. The main ions are lost in the impurity removal process, so a lower concentration of quicklime should be selected. However, the lower quicklime preparation concentration will bring greater challenges to the system water balance and the processing capacity of auxiliary material conveying equipment. Considering comprehensively, it is advisable to choose 15% quicklime preparation concentration.
Figure 1 The influence of quicklime preparation concentration on the impurity removal effect
Figure 2 The effect of quicklime preparation concentration on the loss rate of cobalt ions
2.1.2 Influence of reaction time on impurity removal effect
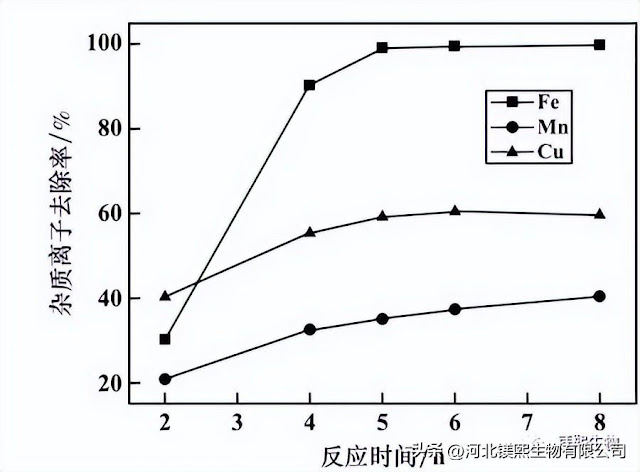
The concentration of quicklime is 15%, the pH=4.0, and the potential is 420mV. The effect of reaction time on the impurity removal effect is shown in Figure 3. It can be seen from Figure 3 that, firstly, with the prolongation of the reaction time, the removal rate of impurity ions gradually increases. The removal rates of iron, copper, and manganese ions tended to be stable at 5h, 4h, and 6h, respectively. Considering comprehensively, it is advisable to choose 5 hours for the reaction time of impurity removal.
Figure 3 The influence of reaction time on the effect of impurity removal
2.1.3 Effect of reaction pH value on impurity removal effect
The influence of pH value on the removal rate of impurity ions is shown in Figure 4 when the concentration of quicklime is 15%, the reaction time is 5h, and the potential is 420mV. It can be seen from Figure 4 that the removal rate of impurity ions in the initial stage of the reaction increases with the increase of pH value. When the pH=3.5, the iron removal rate was 92.3%, and when the pH=4.0, the iron removal rate tended to be stable, maintaining above 99.1%, and continuing to increase the pH value had little effect on iron removal. When pH=4.5, the removal rate of copper ions reaches 60.4%, and when pH=5.5, the removal rate reaches 99.8%. When pH=5.0, the removal rate of manganese ions tended to be stable and maintained at about 41.5%. Excessively high pH value will cause greater loss of cobalt ions. In principle, under the condition of ensuring a good enough impurity removal effect, the lower the pH value, the better. Considering comprehensively, the pH value of impurity removal reaction should be 4.5.
Figure 4 The influence of reaction pH value on the effect of impurity removal
2.1.4 Effect of reaction potential on impurity removal effect
The concentration of...

%20Copper-Cobalt%20Ore%20Activated%20Magnesium%20Oxide.png)









0 Comments