Hebei Messi Biology Co., Ltd. stated that magnesium hydroxide is an important inorganic material and is widely used in daily life. Magnesium hydroxide can be used to neutralize acidic wastewater, adsorb heavy metals in water, act as a decolorizer for wastewater and absorb sulfur dioxide in flue gas. As a green inorganic flame retardant, magnesium hydroxide has received widespread attention at home and abroad. But to make the material achieve the ideal flame retardant effect, the addition of magnesium hydroxide is usually as high as 60%. When its compatibility with organic matter is poor, it will affect the mechanical properties and mechanical properties of the material. The sample has good dispersibility in dry state, which is the prerequisite for good dispersion in organic matter.
The effect of filling conventional magnesium hydroxide and hexagonal flaky magnesium hydroxide on the mechanical properties of ethylene-vinyl acetate copolymer (EVA) was studied, and it was found that the conventional magnesium hydroxide greatly reduced the elongation at break of the material while the hexagonal flaky Magnesium has less of an effect. Highly dispersed hexagonal flaky magnesium hydroxide was prepared by using magnesium chloride and ammonia combined with hydrothermal method, and it was found that the hexagonal flaky product had good compatibility with EVA. Therefore, the preparation of hexagonal flake products with good morphology and dispersion can increase the dispersion and compatibility of magnesium hydroxide in other composite materials, and can also reduce the impact on the mechanical properties and processing properties of materials.
The article studies the effect of the preparation method on the crystal morphology and crystal plane growth. Magnesium hydroxide was synthesized in a microchannel reactor, and the effect of precipitant on crystal morphology and crystal facet growth was explored. Then, coupled with the hydrothermal reaction, the effect of the hydrothermal reaction on the crystal morphology and crystal plane growth was studied, and the process route for preparing magnesium hydroxide for flame retardancy was explored.
1 Experimental materials and preparation methods
1.1 Materials
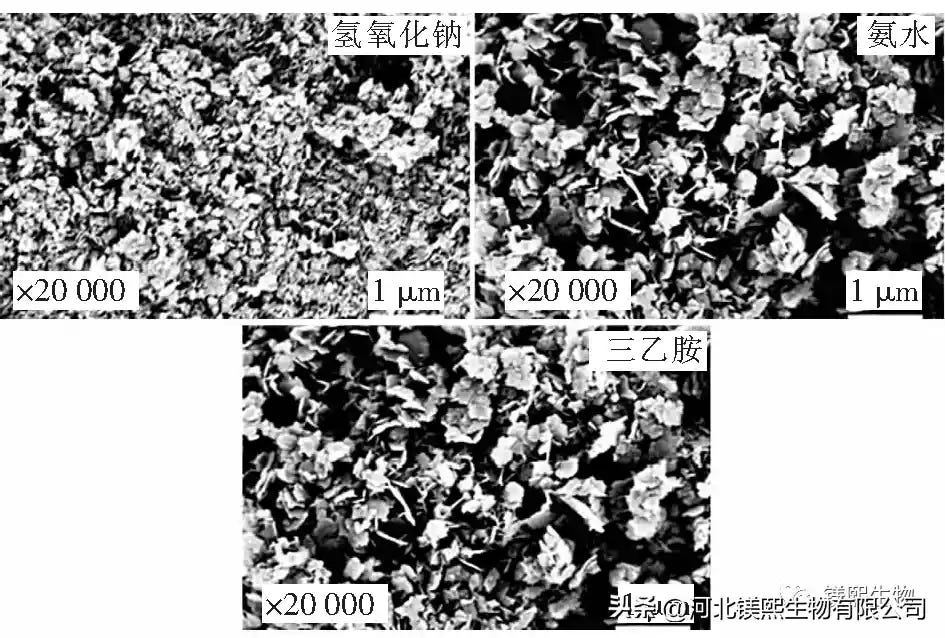
Sodium hydroxide, ethanol and triethylamine, ammonia, brine.
1.2 Reaction device and preparation method
The activated carbon is used to absorb the pretreated brine as the source of magnesium, and the device for preparing magnesium hydroxide using a reactor is shown in Figure 1. Its preparation process is as follows: (1) prepare three kinds of precipitants respectively, wherein the molar ratio of magnesium chloride and precipitant is 1:2; (2) pour the raw material products into the raw material storage tank respectively, and set the constant temperature circulating water bath to 25°C; open The power switch of No. 1 peristaltic pump and No. 2 peristaltic pump, set the same feed flow rate of 50mL/min; (3) Turn on the inlet and outlet switches of the reactor, and after turning on the peristaltic pump, mix the two reactants in the reactor and collect the product (4) After the reaction, the solid sample obtained by centrifuging the slurry was washed three times with deionized water and ethanol; (5) Collect the washed and dried samples for further characterization tests.
Direct hydrothermal preparation process:
https://tibetmag.com/magnesium-hydroxide-for-flame-retardant/





0 Comments